Intellia Therapeutics, Inc. Securities Lawsuit Investigation
Shamis & Gentile P.A., a law firm that advocates for investors who are victims of securities fraud, is investigating potential claims against Intellia Therapeutics, Inc. (NTLA)
If you purchased Intellia Therapeutics, Inc. shares prior to April 3, 2025, and still hold some shares today, you may be able to seek corporate reforms, the return of funds back to the Company, and a court approved incentive award for you, all at absolutely no cost.
About Intellia Therapeutics
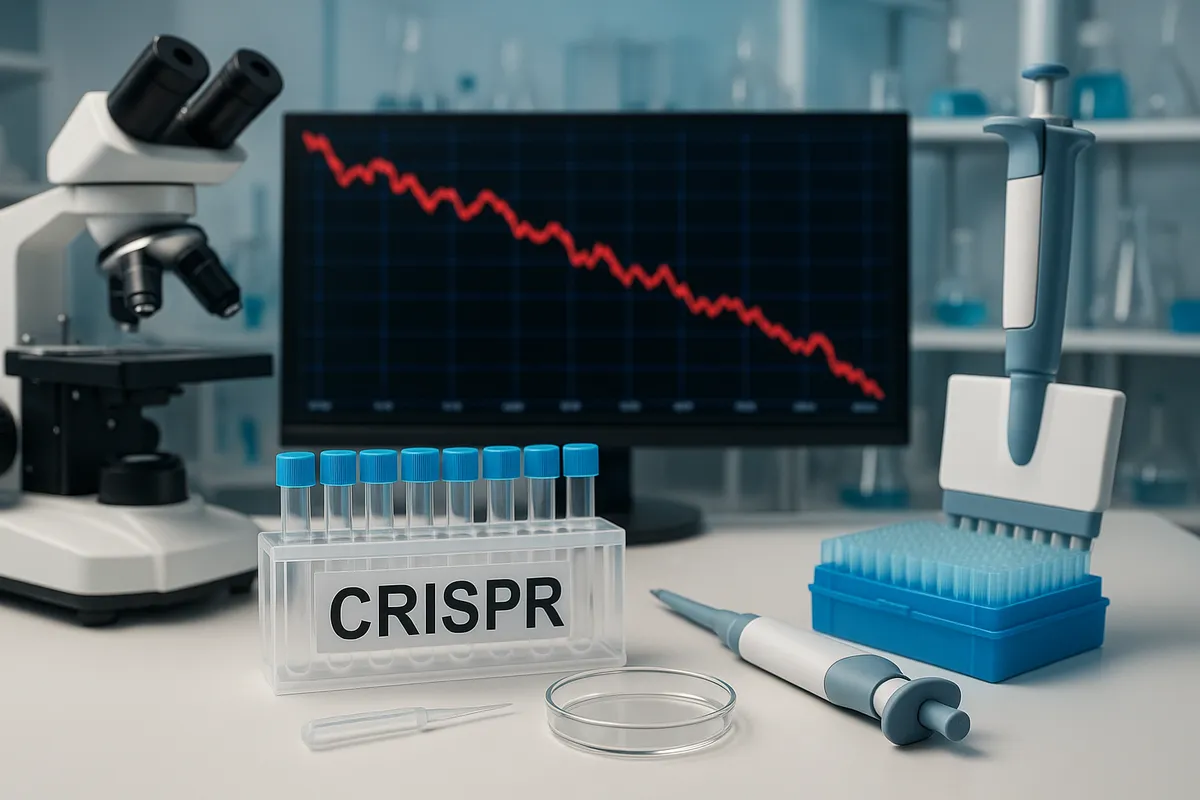
Intellia Therapeutics is a Delaware-incorporated, clinical-stage biotechnology company focused on developing in vivo CRISPR/Cas9 gene-editing therapies. Its programs target hereditary diseases, with lead assets including nexiguran ziclumeran (Nex-Z/NTLA-2001) for ATTR amyloidosis and NTLA-2002 for hereditary angioedema.
What’s Being Investigated
Attorneys are investigating whether Intellia Therapeutics’ directors and officers fulfilled their fiduciary duties in overseeing clinical-trial safety and disclosures, including board-level monitoring of mission-critical risks and regulatory communications during the company’s Phase 3 gene-editing programs.
The review focuses on how Intellia managed and communicated safety developments in its lead CRISPR therapy following reports of severe liver toxicity in a trial participant and the subsequent FDA clinical hold.
Lawyers may also investigate whether management communications between April 3, 2025 and November 6, 2025 accurately reflected trial progress, patient-safety information, the scope of dosing pauses, and the extent of FDA involvement.
Between the first public acknowledgment of the patient’s hospitalization on October 24, 2025 and the day following the disclosure of the death on November 7, 2025, Intellia’s stock declined about 63%, from $25.60 to $9.52.
The inquiry seeks to determine whether Intellia’s leadership exercised proper oversight of trial-safety protocols and external communications, or whether internal incentives and commercial considerations may have contributed to delayed or incomplete reporting.
Key Timeline
- April 3, 2025: Intellia announces first patient dosed in the MAGNITUDE-2 (Phase 3, nex-z) trial for ATTR amyloidosis.
- October 24, 2025: Patient experiences Grade-4 liver transaminase elevations and is hospitalized.
- October 27, 2025: Intellia pauses dosing and screening in both Phase 3 trials.
- October 29, 2025: FDA imposes a verbal clinical hold on both trials.
- November 5, 2025: The patient dies while hospitalized.
- November 6, 2025: Intellia publicly discloses the death and clinical hold.
Your Rights and Next Steps
This is an active investigation into potential breaches of fiduciary duty and oversight failures at Intellia Therapeutics. If you owned Intellia stock during the relevant period and believe you were harmed, you may have important rights.
Possible next steps include:
- Requesting company books and records under Delaware General Corporation Law §220 to investigate potential wrongdoing.
- Participating in a shareholder action on behalf of the company to recover damages or seek governance reforms.
- Monitoring any future regulatory developments or board disclosures about the MAGNITUDE-2 trial and safety oversight.
Shareholder actions seek to hold directors and officers accountable for alleged breaches of fiduciary duty or oversight failures, with any recovery typically benefiting the company. To preserve your rights, it is important to act promptly, as certain deadlines may apply.
What Shareholders Can Do
Shareholder actions of this type are time-sensitive. If you owned Intellia Therapeutics stock during the relevant period and continue to hold shares, you may be eligible to participate in this investigation and seek corporate governance reforms, the return of funds to the company, and a court-approved incentive award at no cost to you.
To participate in the investigation or to learn more about your rights, complete the form below to join the investigation.
.png)



.svg)